-
Microbiome Therapeutics Market Report : By Key Players, Trends, Size, Share and Forecast 2022-2032

Global microbiome therapeutics market is expected to grow further in the coming years due to the continuous increase in awareness and need for better and safer treatment alternatives and the dominance of gastrointestinal (GI) and infectious diseases segment can be attributed to the existing dominance of microbiome therapeutics available for a variety of indications ranging from diarrhea and gut dysbiosis to skin disorders, cancer indications among others. The global microbiome therapeutics market which accounted for $306.3 million in 2021 is expected to reach $3,204 million by 2032, reporting a CAGR of 24.95% during the forecast period 2022-2032.
The existing microbiome therapeutics market is favored by multiple factors, including the rising geriatric populations coupled with the increasing adoption of inorganic growth strategies by key players in the market.
Impact of COVID-19
There were numerous consequences due to the COVID-19 pandemic, especially because of the reduced access to care for other illnesses. As the number of individuals becoming ill from COVID-19 kept increasing and for the protection of healthy individuals from being affected by the disease, all non-urgent healthcare facilities were suspended as per respective government directives. Even though these measures were necessary, it had mostly negative impact on the Global microbiome therapeutics market. Some the major impacts were; interruption of key clinical trial activities, modification of clinical trial guidelines bringing in additional safety measures, and delay in regulatory agencies’ review and approval timeline.
Market Segmentation:
Within the research report, the market is segmented on the basis of:
• target therapy area (gastrointestinal and infectious diseases, skin disorders, cancer indications, and other indications)
• region (North America, Europe, Asia-Pacific, and Rest-of-the-World).This segmentation highlights value propositions and business models useful for industry leaders and stakeholders. The research also comprises country-level analysis, go-to-market strategies of leading players, and future opportunities, among others, to detail the scope and provide a 360-coverage of the domain.

Segmentation 1: by Target Therapy Area
Disorders related to gastrointestinal (GI) and skin disorders command a major share of the microbiome therapeutics segment, and is expected to continue during the forecast period. Most of the microbiome therapeutics in advanced development stage are indicated to treat C.diff. infection (CDI) and expected to enter the market first. The selected indications were on the basis of research intensity in the specific therapy area.
Segmentation 2: by Region
Asia-Pacific (APAC) is expected to dominate the global microbiome therapeutics market for the forecast period 2022-2032 continuing with it overall contribution of approximately 40% of the total market size in 2021 followed by Europe with 32.59%. Increasing healthcare expenditure, coupled with government initiatives, are among the leading factors contributing to the growth of the market.
Demand – Drivers and Challenges
Some of the potential drivers identified by BIS Research include:
• Growing Strategic Activities in Microbiome Therapeutics Segment
• Potential of Microbiome Therapeutics to Address Unmet Needs of Existing Treatment Options
• Microbiome Therapeutic Products as a Safer Alternative to Conventional Drug TreatmentsThere are some challenges identified for the global microbiome therapeutics market are; Lack of standard regulatory guidelines and safety issues associated with live biotherapeutic products (LBPs).
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
The top segment players leading the market include Sanofi SA and Taisho Pharmaceutical Holding that capture around 88% of the market with Sanofi SA holding 64.31% while Taisho Pharmaceutical Holdings 24.49% respectively.

Some of the prominent established names in this market are:
• 4D pharma plc
• Seres Therapeutics, Inc.
• Microbiotics
• Enterome
• Enterome Biosciences
• Destiny Pharma plc
• Taisho Pharmaceutical Holdings
• AOBiome Therapeutics, Inc.
• Finch Therapeutics Group, Inc.
• Rebiotix Inc. (A Subsidiary of Ferring Pharmaceuticals)
• MaaT Pharma
• Vedanta Biosciences Inc.
• OxThera AB
• Pendulum Therapeutics
• Caelus Health
• Quorum Innovations
• Sanofi SA
• DermBiont, Inc.
• EnteroBiotix Ltd
• YSOPIA Bioscience
• Winclove Probiotics
• TargEDys
• Evelo Biosciences, Inc.
• BiomX
• Biomica Ltd.
• Scioto Biosciences, Inc.
• Lactobio A/SKey Questions Answered in the Report
- What are microbiome therapeutics products?
- How has microbiome therapeutics evolved over the years since its inception?
- What are the major market drivers, challenges, and opportunities in the global microbiome therapeutics market?
- What are the potential indications targeted using microbiome therapeutics products?
- What does the pipeline for the microbiome therapeutics products market look like?
- What is the market size and future potential of microbiome therapeutics products?
- How does the clinical trial landscape look for the global microbiome therapeutics products market?
- What was the impact of COVID-19 on this market?
- Which region is expected to contribute the highest revenue to the global microbiome therapeutics products market during the forecast period?
- What are the key development strategies implemented by the major players in order to sustain them in the competitive market?
- How is each segment of the market expected to grow during the forecast period 2022-2032? Following are the segments
- Target Therapy Area (gastrointestinal and infectious diseases, skin disorders, cancer indications, and other indications)
- Region (North America, Europe, Asia-Pacific (APAC), Rest-of-the-World)
- What are the existing partnership landscape and regulations for microbiome therapeutics?
Get Free Sample Report – https://bisresearch.com/requestsample?id=1326type=download
BIS Related Studies
Cancer Microbiome Sequencing Market – A Global and Regional Analysis
Global Skin Microbiome Modulators Market
Global Human Microbiome Modulators Market – Analysis and Forecast, 2018-2023
-
Digital PCR Market to Witness Revolutionary Growth by 2032

Increasing investments in R&D for d-PCR molecular diagnostics is one of the major opportunities in the global digital PCR market. Several biotechnology and life sciences companies are working collaboratively on diagnostic test development and using d-PCR as a therapeutic means for applications in several disease indications to enable efficient diagnosis, treatment selection, and treatment monitoring.
The global digital PCR market is projected to reach $2,847.8 million by 2032 from $441.2 million in 2021, at a CAGR of 18.73% during the forecast period 2022–2032. The growth in the global digital PCR market is expected to be driven by factors such as the increasing prevalence of infectious and genetic diseases globally, the rising awareness of d-PCR-based diagnostic testing, and the significant number of funding for executing research and development.
Impact of COVID-19
The current global digital PCR market comprises kits and assays, system, and reagents and consumables. The unexpected crisis of the pandemic has surged the demand for essentials and increased the demand for digital PCR products. There was increased demand for digital PCR from biotechnology and life sciences companies. Overall, the impact of COVID-19 on the global d-PCR market size has been low-moderate. Some market players have reported a slight decline in sales. However, some new entrants in the global d-PCR market are expected to witness growth in the market. There is also a decline in revenues mostly due to the initial phases of the COVID-19 pandemic, which comprised complete lockdowns across countries and major cities, thereby interrupting the supply chain. Several other products have been under development for years that may take longer than 2–4 years for commercialization. The pandemic has played a key role in enhancing the growth prospects of digital PCR-based diagnostics and is expected to indirectly aid in improving the market growth outlook.
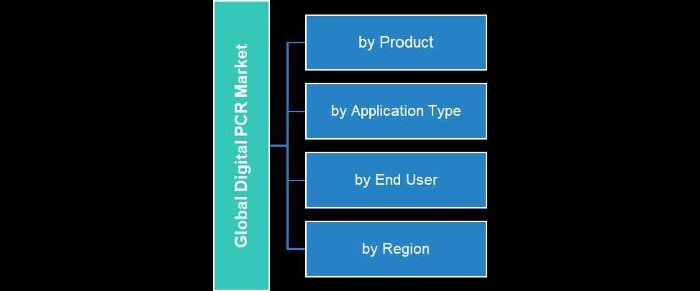
The BIS Research report on the global digital PCR market segments the market based on the following:
- Product (systems, kits and assays, reagents and consumables)
- Application (oncology, infectious disease, rare diseases, reproductive genetics, and other applications),
- End user (academic and research institutions, diagnostics centers, hospitals and clinics and biopharmaceutical companies)
- Region (North America, Europe, Asia-Pacific, Latin America, and Rest-of-the-World).
Recent Developments in the Global Digital PCR Market
• In March 2022, Stilla signed an agreement with 12 distributors throughout EMEA. This agreement grants distribution rights to Stilla’s full product portfolio, including the six-color naica system.
• In April 2022, Stilla and Promega Corporation signed a co-marketing agreement to offer a complete digital PCR workflow solution to provide their end users with optimized workflow for a wide range of applications, including liquid biopsy, sentinel pathogen testing, infectious disease assay, overall cancer research, and drug discovery.
• In October 2021, Bio-Rad Laboratories, Inc. acquired Dropworks, Inc., a development stage company focused on developing a digital PCR product. The strategy was to increase and complement the company’s life sciences product offerings.
• In September 2021, Thermo Fisher Scientific launched the Applied Biosystems QuantStudio Absolute Q Digital PCR System, the first fully integrated digital PCR (dPCR) system designed to provide highly accurate and consistent results.Digital PCR Market Segmentation
Demand — Drivers and Limitations
Following are the demand drivers for the global digital PCR market:
• Technological Transformations Related to Digital PCR
• Rising Adoption of Digital PCR among Various Applications
• Increasing Activities in d-PCR EcosystemThe market is expected to face some limitations too due to the following challenges:
• Higher Cost of Droplet Digital PCR
• Presence of Opaque Regulatory FrameworkKey Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
Some prominent names established in this market are:
• Bio-Rad Laboratories, Inc.
• Fluidigm Corporation
• F. Hoffmann-La Roche Ltd.
• Merck KGaA
• QIAGEN N.V.
• Thermo Fisher Scientific Inc.
• Genetron Holdings Limited
• JN Medsys
• Stilla
• Naveris, Inc.
• SAGA DiagnosticsGet Free Sample Report — https://bisresearch.com/requestsample?id=1348type=download
Key Questions Answered in the Report
- How is digital PCR revolutionizing the field of genomics?
- What are the major market drivers, challenges, and opportunities in the global digital PCR market?
- What are the underlying structures resulting in the emerging trends within the global digital PCR market?
- How did the COVID-19 pandemic impact the global digital PCR market?
- What are the key development strategies that are being implemented by the major players in order to sustain themselves in the competitive market?
- What are the key regulatory implications in developed and developing regions pertaining to the use of digital PCR?
- What are the potential entry barriers that are expected to be faced by the companies willing to enter a particular region?
- How is each segment of the market expected to grow during the forecast period 2022–2032, and what is the anticipated revenue to be generated by each of the segments?
BIS Related Studies
Molecular Diagnostics Market — A Global and Regional Analysis
-
Radiation Dose Management Market Analysis, Key Company Profiles, Types, Applications and Forecast to 2032

Radiation dose is the amount of radiation absorbed by a patient during a diagnostic imaging procedure. A large dose of radiation can cause tissue damage and increase the risk of developing cancer in later stages of life.The development of on-cloud dose management solutions and the growing initiatives on radiation dose management for pediatric procedures are some of the major opportunities in the global radiation dose management market. Furthermore, some of the key trends going on in the market include the increasing development of imaging modalities with technologies that lower radiation dose, partnerships and strategic business alliances dominating the market, and various collaborations among market players.
The global radiation dose management market was valued at $212.8 million in 2021 and is anticipated to reach $586.7 million by 2032, witnessing a CAGR of 10.30% during the forecast period 2022–2032. The growth in the global radiation dose management market is expected to be driven by the rising prevalence of chronic disorders requiring diagnostic intervention, the growing focus on interventional radiology (IR), and the growing awareness surrounding patient safety and the harmful and biological effects of overexposure to ionizing radiation.

Impact of COVID-19
The decline in imaging volumes during the COVID-19 pandemic impacted the utilization of radiation diagnostic equipment. All radiology equipment had to be thoroughly disinfected using ethanol owing to the strict protocols to minimize the risk of transmission of COVID-19.
In the post-COVID-19 scenario, imaging volumes are expected to return gradually to pre-pandemic levels. As the severity of the COVID-19 pandemic declines, hospitals are expected to reallocate resources toward radiology and pursue diagnostic imaging with renewed vigor. Moreover, in the long term, radiology practices could be permanently redesigned as radiologists become more comfortable treating patients remotely. Furthermore, hospitals and other healthcare institutions are expected to be better equipped to deal with unprecedented events that may disrupt their businesses.
The COVID-19 pandemic placed high importance on automating tasks requiring manual intervention. While conventionally, data transfer and dose recording were done manually, the advent of advanced dose management software (DMS) has enabled automated data transfer from imaging systems into the DMS as well as automated dose recording. This helps eliminate human error and reduces the need for human intervention, which was a concept that gained momentum as a result of the COVID-19 pandemic.
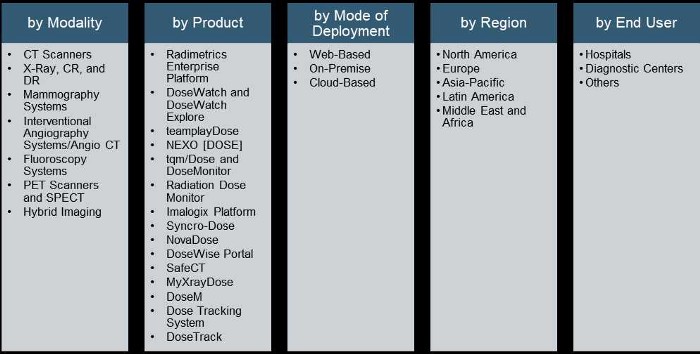
Market Segmentation
Recent Developments in the Global Radiation Dose Management Market
• In December 2020, Bayer AG launched Radimetrics v3.0, the latest evolution of its enterprise dose management utility.
• In December 2020, FUJIFILM Holdings Corporation launched the unique SYNAPSE Dose 2.0 to provide a comprehensive system for managing patient radiation exposure across several imaging modalities.
• In January 2020, Agfa HealthCare collaborated with Maasstad Hospital in the Netherlands to support advancements in the consistency and optimization of radiation doses.
• In April 2019, Qaelum NV signed an agreement with TOYO Corporation to distribute its dose monitoring solution, DOSE, in Japan.
• In January 2019, SST Group, Inc. partnered with Intermountain Healthcare to implement a software platform for the Radiation Dose Monitor (RDM). The software platform permits medical professionals to manage, examine, and optimize the dose delivered to a patient during interventional procedures, medical imaging examinations, and image-guided surgery.
• In August 2018, Imalogix launched a newer version of its radiation dose management platform with additional fluoroscopy capabilities. The software solution can automatically calculate the peak skin dose for the patient during a fluoroscopic procedure.
• In August 2018, Guerbet LLC USA, a subsidiary of the Guerbet Group, partnered with Imalogix to decrease inconsistency and advance the quality, safety, and competence of care around radiation dose management, to enable the network health system and member facilities to monitor patient radiation dose on a per-procedure and cumulative basis.
• In March 2018, PACSHealth, LLC launched DoseMonitor, a radiation dose management software for tracking radiation dose absorption.Demand — Drivers and Limitations
Following are the drivers for the global radiation dose management market:
• Increasing installed bases of radiology equipment and the number of scans lead to a higher risk of radiation exposure, thus creating demand for dose management solutions.
• The increasing prevalence of cardiovascular diseases and growing occupational hazards in cath labs lead to the growing concern surrounding the effects of radiation exposure.
• Growing focus on interventional radiology (IR) is expected to increase demand for IR imaging systems and subsequently lead to a higher risk of radiation exposure.
• Increasing concern related to radiation overexposure is leading to higher adoption of dose management solutions.
• Growing awareness and initiatives for radiation dose management are expected to push the adoption of dose management software.
• Advancements in dose optimization and benchmarking in various countries will encourage healthcare institutions to adopt dose management software.The market is expected to face some limitations as well due to the following challenges:
• The lack of awareness among the population and the shortage of skilled and trained professionals leading to a steep learning curve hinders the adoption of dose management software.
• The lack of diagnostic reference levels (DRLs) for radiation dose benchmarking in low-income countries causes healthcare institutions to not pursue the adoption of dose management software with vigor.Check out the detailed table of content here: — https://bisresearch.com/requestsample?id=1409type=toc
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
Some of the prominent names established in this market are:
• Agfa-Gevaert Group
• Bracco
• Bayer AG
• Canon Inc.
• FUJIFILM Holdings Corporation
• Guerbet
• General Electric Company
• Imalogix
• Infinitt Healthcare Co., Ltd.
• Koninklijke Philips N.V.
• LANDAUER
• Medic Vision Imaging Solutions Ltd.
• Medsquare
• MyXrayDose Ltd.
• Novarad Corporation
• PACSHealth, LLC
• Qaelum NV
• Sectra AB
• SST Group Inc.
• Siemens Healthineers AG
• Virtual Phantoms Inc.Companies that are not a part of the previously mentioned pool have been well represented across different sections of the report (wherever applicable).
Get Free Sample Report — https://bisresearch.com/requestsample?id=1409type=download
The USP of this report
The following can be considered USPs of this report:
· Detailed impact of COVID-19 on the global radiation dose management market
· Gap analysis
· Price analysis
· Detailed segmentation based on product with in-depth information on each of the 15 dose management software
· Market share analysis of the major players done to offer a holistic view of the global radiation dose management market landscape
Who should buy this report?
The companies manufacturing and commercializing radiation dose management software for use in hospitals, diagnostic centers, research institutes, and ambulatory and specialty clinics should buy this report. Moreover, companies who are into dosimetry systems and looking to venture into software solutions for radiation dose management can also buy this report
-
Molecular Oncology Diagnostics Market Statistics, Segment, Trends and Forecast to 2032

BIS Research, the global leader in providing market intelligence on deep technologies, has released its latest study titled Molecular Oncology Diagnostics Market – A Global and Regional Analysis.
According to this study, the market for global molecular oncology diagnostics was valued at $3.62 billion in 2021 and is projected to reach $12.13 billion by 2032.
The following factors are responsible for the increase in demand for molecular oncology diagnostics:
- Rising incidence of cancer cases
- Launch of innovative products in the molecular oncology diagnostics ecosystem
- Growth in biomarker identification and transformations in molecular techniques
The detailed study is a compilation of 3 market data tables and 206 figures spread through 264 pages.
Check out the detailed table of content here
https://bisresearch.com/requestsample?id=1402type=toc
Analyst’s Take on the Market Projection
According to Nitish Kumar, Principal Consultant, Healthcare, BIS Research, “Although there have been significant innovations for the enhancement of all the important areas of diagnostics, molecular diagnostics, particularly, has harbored phenomenal attention in recent years owing to the in-depth insights they bring to diagnosis and treatment. As the next frontier of precision medicine, the global molecular oncology diagnostics market is expected to showcase enormous potential to completely revolutionize patient care and overall wellness in general.”
Impact of COVID-19
In response to the COVID-19 pandemic and the impact on medical research in the U.S., the National Institutes of Health (NIH) and the National Cancer Institute (NCI) both extended grant application deadlines, laid back reporting requirements, and offer flexibility in how to grant money is spent. COVID-19 drastically reduced fundraising possibilities for cancer-focused charitable research organizations, which provide up to half of all cancer research funding in the U.S. and frequently fund high-risk, high-reward projects even though the national government supported research work and offered medical researchers the flexibility to implement their skill set and knowledge to studying SARSCoV-2.
Request a FREE sample of this report here
https://bisresearch.com/requestsample?id=1402type=download
Existing Competitive Landscape
The companies that are profiled have been selected based on input gathered from primary experts and analyzing company coverage, product portfolio, and market penetration. Some of the established names in the market are Agilent Technologies, Inc., Abbott, Biocartis NV, Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd., QIAGEN N.V., Thermo Fisher Scientific, Inc., Danaher, Guardant Health, HTG Molecular Diagnostics, Inc., Illumina, Inc., Invivoscribe, Inc., Myriad Genetics, Inc., Sysmex Corporation, and more.
Recent Developments in the Global Molecular Oncology Diagnostics Market
- In August 2022, Guardant Health expanded its Guardant Reveal usage, a liquid biopsy test for residual disease detection and recurrence monitoring, to include early-stage breast and lung cancers. It is the only tissue-free test used for colorectal cancer (CRC) and is now available for patients with breast and lung cancer.
- In December 2021, QIAGEN partnered with Denovo Biopharma to develop a companion diagnostic test for the treatment of diffuse large B-cell lymphoma. This test identified patients expressing Denovo genomic marker 1 (DGM1), which responded to Denovo’s investigational cancer drug DB102.
- In September 2021, Illumina partnered with Merck to develop and commercialize research tests for use in identifying specific cancer genetic mutations used in the assessment of homologous recombination deficiency (HRD).
- In August 2021, Agilent Technologies, Inc. expanded its CE-IVD marked PD-L1 IHC 22C3 pharmDx assay usage in Europe. This assay was used as an aid in identifying esophageal cancer patients for treatments with KEYTRUDA using a combined positive score.
Key Questions Answered in the Report
- What are the major market drivers, challenges, and opportunities and their respective impacts in the global molecular oncology diagnostics market?
- What are the underlying structures resulting in the emerging trends within the global molecular oncology diagnostics market?
- What is the current market demand, along with future expected demand for the global molecular oncology diagnostics market?
- What are the key development strategies that have been implemented by the major players in order to sustain the competitive market?
- Which potential entry barriers are expected to be faced by the companies willing to enter the global molecular oncology diagnostics market?
Following are the demand drivers for the global molecular oncology diagnostics market:
• Rising Incidence of Cancer Cases
• Launch of Innovative Products in Molecular Oncology Diagnostics Ecosystem
• Growth in Biomarker identification and Transformations in Molecular TechniquesThe market is expected to face some limitations due to the following challenges:
• Lack of Qualified Professionals
• Opaque Regulatory Framework Delaying the Approval of New Molecular Diagnostic Tests
• High Cost of Equipment Hindering the Adoption RateKey Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts, analyzing company coverage, product portfolio, and market penetration.
Some of the prominent names in this market are:
• Agilent Technologies, Inc.
• Abbott.
• Biocartis NV
• Bio-Rad Laboratories, Inc.
• F. Hoffmann-La Roche Ltd.
• QIAGEN N.V.
• Thermo Fisher Scientific, Inc.
• Danaher.
• Guardant Health
• HTG Molecular Diagnostics, Inc.
• Illumina, Inc.
• Invivoscribe, Inc.
• Myriad Genetics, Inc.
• Sysmex CorporationPolymerase Chain Reaction Technology Type to Witness the Highest Market Growth
The global market for molecular oncology diagnostics (by technology) has been divided into six categories, i.e., next-generation sequencing (NGS), polymerase chain reaction (PCR), fluorescence in-situ hybridization (FISH), immunohistochemistry (IHC), flow cytometry, and others.
These rapidly evolving technologies have the potential to transform medical diagnoses and treatment for the better. Not only are developing diagnostic tools becoming more useful in a wider range of clinical scenarios, but they are also assisting in a more exploratory outlook in order to cater to a holistic approach to cancer treatment.
According to this study from BIS Research, the PCR segment dominated the technology market in 2021 with a cumulative value of $1.20 billion, and the trend is expected to continue during the forecast period 2022-2032.
Want to learn more about the latest trends in precision medicine? Speak to our analysts
Exclusive DeepTech™ MAP Analysis for Healthcare by BIS Research:
-
In-Silico Drug Discovery Market Drivers, Threats, and Opportunities between 2021-2031

In-silico drug discovery methods can help in identifying drug targets via bioinformatics tools. In addition, the methods can also be used to analyze the target structures for possible binding active sites. The use of computational methods and computers permeates all aspects of drug discovery and drug design. The methods are facilitating the access of massive amounts of data generated and transforming the extensive complex biological data into useful knowledge for the drug discovery process. These computational tools offer the advantage of delivering new drug candidates more quickly and at a lower cost.
BIS Research’s healthcare experts have found the In-silico drug discovery market to be one of the stable markets, and the global market is predicted to grow from $2,129.8 million in 2020 to $6,515.3 million in 2031 and is expected to grow with a CAGR of 10.52% during the forecast period 2021-2031.
Factors fueling the growth of the market include the rising emphasis on reduction in medical errors, technological advancements in the field of computational biology, and the rising adoption of cloud-based applications in drug discovery procedures. New drug compounds have been developed using computational methods successfully. The developments in computational biology have eased the data analytics and analysis phases of sequencing and have resulted in fewer turnaround times and greater accuracy.
Market Segmentation
• Workflow – Discovery, Pre-Clinical Tests, and Clinical Trials
• Products – Software, Software-as-a-Service (SaaS), and Consultancy-as-a-Service
• Technology – Artificial Intelligence, Graphics Processing Unit (GPU), and Other Technologies
• Software – Molecular Modeling and De Novo Drug Design Software, Pharmacophore Modeling Software
• End User – Contract Research Organizations, Pharmaceutical and Biopharmaceutical Companies, Academic and Research Institutes, and Other End Users.Regional Segmentation
• North America: U.S., Canada
• Europe: Germany, France, Italy, U.K., Spain, Russia, and Rest-of- Europe
• Asia-Pacific: Japan, China, India, Australia, South Korea, and Rest-of-Asia-Pacific
• Latin America: Brazil, Mexico, Rest-of-Latin America
• Rest-of-the-World
Market Growth Drivers
• Rising Emphasis on Reduction in Medical Errors
• Technological Advancements in the Field of Computational Biology
• Integration of Blockchain Technology in Interoperability
• Rising Adoption of Cloud-Based Application for Drug Discovery
Market Challenges
• Lack of High Complexity Testing Centers
• Expensive In-Silico Drug Discovery Procedures and Decline in the Number of Approved Drugs
• High Capital Requirement Hampering the Expansion of Global Reach
Market Opportunities
• Massive Scope for Adoption of In-Silico Drug Discovery in Developing Nations
• Adoption of Artificial Intelligence in In-Silico Drug DiscoveryCompetitive Landscape
The exponential rise in the prevalence of infectious diseases and various types of cancer globally has created a buzz among companies to invest in advanced technologies such as artificial intelligence.
Based on region, North America holds the largest share, owing to improved healthcare infrastructure, rise in per capita income, and improvised reimbursement policies in the region. However, the Asia-Pacific and Europe regions are anticipated to grow at the fastest CAGR during the forecast period.
Key Companies Profiled
Aragen Life Sciences Pvt. Ltd., Curia Global, Inc., Certara, USA., Charles River, Chemical Computing Group ULC., Collaborative Drug Discovery Inc., Dassault Systemes, e-therapeutics plc., Evotec, Insilico Medicine, Ligand Pharmaceuticals Incorporated, Numerate, Inc., PerkinElmer Inc., Schrödinger, Inc., Selvita, Simulations Plus, Tracxn Technologies, WuXi AppTecKey Questions Answered in this Report:
• How is each segment of the market expected to grow during the forecast period 2021-2031, and what is the anticipated revenue to be generated by each segment? Following are the segments:
o Workflow (Discovery, Pre-Clinical Tests, and Clinical Trials)
o Products (Software, Software-as-a-Service (SaaS), and Consultancy-as-a-Service)
o Technology (Artificial Intelligence, Graphics Processing Unit (GPU), and Other Technologies)
o Software (Molecular Modeling and De Novo Drug Design Software, Pharmacophore Modeling Software)
o End User (Contract Research Organizations, Pharmaceutical and Biopharmaceutical Companies, Academic and Research Institutes, and Other End Users)
o Region (North America, Europe, Asia-Pacific, Latin America, and Rest-of-the-World)
• What are the major market drivers, restraints, and opportunities in the global in-silico drug discovery market?
• What are the underlying structures resulting in the emerging trends within the global in-silico drug discovery market?
• How is each segment of the global in-silico drug discovery market expected to grow during the forecast period, and what will be the expected revenue generated by each of the segments by the end of 2031?
• What are the key developmental strategies implemented by the major players to sustain in the competitive market?
• What are the key regulatory implications in developed and developing regions for in-silico drug discovery?
• Who are the leading players with significant offerings to the global in-silico drug discovery market? What is the current market dominance for each of these leading players?
• What would be the compound growth rate witnessed by the leading players in the market during the forecast period 2021-2031? Which in-silico drug discovery workflow type has the most promising growth?
• What are the major technologies that are employed in the global in-silico drug discovery market? Which is the dominating technology?
• Who are the primary end users of the global in-silico drug discovery market? Which is the fastest-growing end-user segment in the global in-silico drug discovery market?
• Who are the key manufacturers in the global in-silico drug discovery market, and what are their contributions? Also, what is the growth potential of each major in-silico drug discovery manufacturer?
• What is the scope of the global in-silico drug discovery market in North America, Europe, Asia-Pacific, Latin America, and Rest-of-the-World? Which in-silico drug discovery technology and end user dominate these regions?
• What are the emerging trends in the global in-silico drug discovery market? How are these trends revolutionizing the diagnostic procedure?
• Which technologies are anticipated to break through the in-silico drug discovery regime?
• Which companies are anticipated to be highly disruptive in the future and why?
• Which regulatory procedures are required to unify the approval process for the emerging in-silico drug discovery market? How will these enhance the reimbursement scenario?
• What are the gaps in regularizing in-silico drug discovery adoption in regular healthcare routines? How are these gaps being tackled?Request Sample – https://bisresearch.com/requestsample?id=1252type=download
Within the research report, the market has been segmented based on workflow, product, technology, software type, end user, and region. Each of these segments covers the snapshot of the market over the projected years, the inclination of the market revenue, underlying patterns, and trends by using analytics on the primary and secondary data obtained.
BIS Related Studies
-
Neoantigen Cancer Vaccine Market is Expected To Grow at the Highest CAGR of 77.73% During 2024-2031

Looking at the recent oncology care trends, the World Heart Foundation estimates the global cancer treatment expenditure to reach $XX billion in the year 2031. The increase in overall burden provides an opportunity for several companies to step in and introduce their innovative products in the market. The emergence of the neoantigen cancer vaccine can be considered as an evident example of emerging oncology care that can possibly prevail over the existing hurdles of treatment un-specificity and safety benchmarks.
The global neoantigen cancer vaccine industry analysis by BIS Research projects the market to grow at a significant CAGR of 77.73% during the forecast period 2024-2031. The neoantigen cancer vaccine market is expected to generate $35.5 million in revenue in 2024, owing to the expected launch of the first neoantigen vaccine, DC vaccine in the market.
The neoantigen cancer vaccine market growth has been primarily attributed to major drivers in this market, such as rising prevalence of cancers, increasing adoption of personalized medicine to tailor patient’s treatment on an individual level, and significant external funding for executing research and development exercises. However, significant challenges are restraining the market growth. These challenges include the expected higher cost of personalized cancer vaccines, hurdles in clinical development, and payer uncertainty and outcome-based pricing.
The global neoantigen cancer vaccine market has been analyzed on the basis of segments mentioned in the following figure
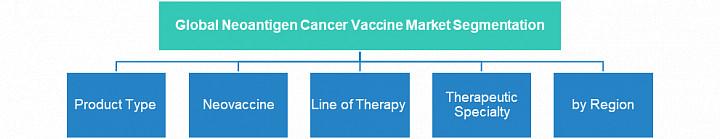
Pipeline Segmentation
• The emerging neoantigen cancer vaccines are segmented based on product type into personalized and off-the-shelf neovaccines.
• The emerging neoantigen cancer vaccines are segmented based on disease/application into lung cancer, urinary system cancer, melanoma, liver cancer, head and neck cancer, and blood and bone marrow cancer.
• The emerging neoantigen cancer vaccines are segmented based on type of neovaccine into nucleic acid vaccine, peptide vaccine, and dendritic cell vaccine.
• The emerging neoantigen cancer vaccines are segmented based on line of therapy into first, second, and later lines therapy.
• The emerging neoantigen cancer vaccines are segmented based on development phase into Phase I, II, and III.
• The emerging neoantigen cancer vaccines are segmented on the basis of route-of-administration into intradermal, subcutaneous, and intramuscular administrations.Expert Quote on the Neoantigen Cancer Vaccine Market
“The real issues with neovaccines are higher cost, turn-around times, and limited efficacy. The cost and time to manufacture have come down in the last four years, and as technology improves, it will come down more.”
The neoantigen cancer vaccine market report provides a holistic view of the market in terms of various factors influencing it, including reported clinical findings, financing and partnership opportunities, expected market, and current clinical landscape.
The scope of this report is centered upon conducting a detailed study of the products expected to be allied with the oncology market. In addition, the study also includes exhaustive information on the unmet needs, perception on the new products, competitive landscape, market share of key players, growth potential of each underlying sub segment and company, as well as other vital information with respect to the global neoantigen cancer vaccine market.
Market Growth Drivers
• Increasing Global Prevalence of Cancer
• Increase in Adoption of Personalized Medicine to Tailor Patient’s Treatment on an Individual Level
• Significant External Funding for Executing Research and Development Exercise
Market Challenges
• Higher Cost of Personalized Cancer Vaccines
• Hurdles in Clinical Development and Optimization Process
• Uncertain Reimbursement Scenario
• Payer Uncertainty and Outcome-Based Pricing
• High Capital Requirement Hampering the Expansion of Global Reach
Market Opportunities
• Treatment Gaps
• Reduced Turnaround Time and Cost
• Partnerships and Collaboration between Various Healthcare Stakeholders
• Data Analytics
Key Companies Profiled
Moderna Therapeutics, F. Hoffmann-La Roche Ltd, AstraZeneca plc, Agenus, OSE Immunotherapeutics, Advaxis, Medigene, Neon Therapeutics (Acquired by BioNTech SE), Genocea Biosciences, Immunovative Therapies, Gritstone Oncology, Nouscom, NantBioScience, Immunovaccine, BioLineRx, Geneos Therapeutics.Key Questions Answered in this Report:
• What are the major market drivers, challenges, and opportunities in the global neoantigen cancer vaccines market?
• What are the underlying structures resulting in emerging trends within the global neoantigen cancer vaccines market?
• What key development strategies are implemented by the major players in order to sustain in the competitive market?
• What are the key regulatory implications in developed and developing regions for neoantigen cancer vaccines?
• How each segment of the market is expected to grow during the forecast period 2024-2031, and what is the estimated revenue to be generated by each of the segments on the basis of:
o Product Type (Personalized and Off-the-Shelf),
o Type of Neovaccine (Nucleic Acid Vaccine, Peptide Vaccine, and Dendritic Cell-Based Vaccine)
o Line of Therapy (First Line, Second Line, and Later Lines)
o Region, including North America, Europe, Asia-Pacific and Middle East
• Who are the leading players with significant offerings to the global neoantigen cancer vaccines market? What is the expected market dominance for each of these leading players?
• Which companies are anticipated to be highly disruptive in the future and why?
• What are the current treatment gaps, and how neovaccines are expected to fill these gaps?
• What are the unmet needs in the global neoantigen cancer vaccine market?Request Sample – https://bisresearch.com/requestsample?id=1254type=download
The report scope exclusively covers biotechnology companies actively engaged in the development of Neoantigen Cancer Vaccine. The contract research and manufacturing organizations for neoantigen prediction, optimization, and vaccine manufacturing have not been considered as a part of the report.
BIS Related Studies
-
Serum-Free Media Market Global Key Findings, Industry Demand, Regional Analysis, & Key Players Profiles

Global serum-free media market has evolved to be an integrative aspect of healthcare procedures by providing most diagnostic laboratory tests equipped with infectious disease, oncology, and genetics. The massively parallel methods that have now transcended media-based cellular diagnostics further allow the sequencing of entire genomes at very low costs and are expected to grow further in the coming years due to the continuous increase in prognostic assessments for a wide range of diseases.
The global serum-free media market was valued at $1,022.4 million in 2021 and is expected to reach $3,920.5 million by 2032, registering a CAGR of 12.73% during the forecast period 2022-2032. The growth in the global serum-free media market is expected to be driven by factors such as the low risk of contamination in the media, rising awareness of media-based diagnostic testing, and significant number of funding for executing research and development.

Recent Developments in the Global Serum-Free Media Market
• In August 2022, Thermo Fisher Scientific Inc. expanded the cell culture media manufacturing facility in Grand Island, New York, to support the production of new vaccines and biologics. This expansion would support the worldwide media supply and increase the capacity to create high-quality technologies.
• In May 2022, FUJIFILM Irvine Scientific completed its new bioprocessing innovation and collaboration center in China. The new center would enable local resources and expertise required to optimize cell culture media and workflows to aid the production of vaccines, advanced therapies, and biotherapeutic drug development in China.
• In September 2021, Bio-Techne Corporation developed a new medium ExCellerate IpsC expansion medium, for IpsC research in regenerative medicine.
• In April 2021, PromoCell GmbH launched an upgrade to its Keratinocyte Cell Culture Portfolio. The company expanded its improved animal-extract-free Keratinocyte Growth Medium 3 for the standardized isolation and development of juvenile and adult Normal Human Keratinocytes.
• In March 2021, the company also launched its improved third-generation Melanocyte Cell Culture Portfolio. Melanocyte Growth Medium M3 is a serum-free, bovine pituitary extract (BPE)-free, and phorbol myristate acetate (PMA)-free media formulation.Demand – Drivers and Limitations
Following are the demand drivers for the global serum-free media market:
• Low Risk of Contamination as a Demand Driver
• Launch of innovative products in Serum-Free Media Ecosystem
• Low reception cost involving small and medium enterprises in comparison to animal derived mediaThe market is expected to face some limitations too due to the following challenges:
• Slow Growth Rate in Comparison to Serum-Supplemented Media
• Requirement of Diverse Cell-Type for Specific Media FormulationKey Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.

Some of the prominent names established in this market are:
Company Type 1: Serum-Free Media Private Industry
• MP BIOMEDICALS.
• HiMedia Laboratories
• RD Systems, Inc.
• Sino Biological, Inc.
• PAN-Biotech.
• PromoCell GmbH
• Athena Environmental Sciences, Inc.
• STEMCELL Technologies Inc.Company Type 2: Serum-Free Media Public Industry
• Corning Incorporated
• Lonza Group AG
• Merck KgaA
• Sartorius AG
• Thermo Fisher Scientific Inc.
• FUJIFILM Holdings Corporation
• Danaher.Get Free Sample Report – https://bisresearch.com/requestsample?id=1361type=download
Key Questions Answered in Report
- What is the role of media type in serum-free media, and what are the advantages associated with serum-free media? What is the importance of recombinant proteins in cell culture applications?
- What are the key trends in the global serum-free media market? How is the market evolving, and what is its future scope?
- What are the major drivers, challenges, and opportunities of the global serum-free media market?
- What are the key developmental strategies implemented by the key players in the global serum-free media market to sustain the competition of the market? What is the percentage share of each of the key players in different key developmental strategies?
- What is the regulatory scenario of the global serum-free media market? What are the initiatives implemented by different governmental bodies and guidelines put forward to regulate the commercialization of serum-free media products?
BIS Related Studies
-
Phosphoramidite Market Latest Trend, Top Companies, Regional Analysis and Forecast Report

Global Phosphoramidite Market: Industry Overview
The global phosphoramidite market is projected to reach $2,062.9 million by 2032 from $900.3 million in 2021, at a CAGR of 7.78% during the forecast period 2022-2032. The market growth can be ascribed to the rising demand for oligonucleotides, growing synthetic biology market, increasing partnerships and collaborations, continuous investment for research and development activities by private and public firms, and growing prevalence of several diseases creating an urgent need for novel therapeutic treatments.

Recent Developments in the Global Phosphoramidite Market
• In August 2019, the company acquired 75% ordinary capital of the parent company of Toronto Research Chemicals Inc., i.e., Biomed Pharmaceutical Limited.
• In July 2022, TriLink BioTechnologies expanded its RD leadership team’s responsibility for leading the company’s scientific direction and research and development strategy.
• In May 2022, TriLink BioTechnologies announced a cooperative agreement with the Department of Defense and included funding for TriLink’s expansion in San Diego, California, for nucleic acid production.
• In November 2021, Biosynth Carbosynth acquired Kexing Biopharmaceutical Co., Ltd.’s raw material manufacturing unit located in Jinan, China, to expand its operations and deliver a wide range of products at a larger production volume and increased capacity. The facility extends to more than 30,000 square meters of area.
• In November 2021, a leading investment firm, KKR, invested an undisclosed amount and acquired Biosynth Carbosynth from its founders, Armira and Dr. Urs Spitz. However, senior management will retain a significant ownership stake, and KKR will contribute as a strategic partner. KKR plans to encourage and accelerate the company’s product portfolio expansion and geographic buildout and widen its capabilities.Global Phosphoramidite Market Segmentation
Demand – Drivers and Limitations
Following are the demand drivers for the global phosphoramidite market:
• Increasing Synthetic Nucleotide Applications in Therapeutics
• Growth in Synthetic BiologyThe market is expected to face some limitations due to the following challenges:
• Challenges in Developing Long Nucleotide Sequences
• Competition from Emerging DNA Synthesis TechnologiesKey Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing the company’s coverage, product portfolio, and market penetration.
Some of the prominent names in this market are:
• Biosynth Carbosynth
• Bioneer Corporation
• Lumiprobe Corporation
• QIAGEN N.V.
• Danaher Corporation (IDT)
• TriLink BioTechnologies
• Creative Biolabs
• PolyOrg, Inc.
• Hongene Biotech Corporation
• ChemGenes
• BOC Sciences
• Tokyo Chemical Industry Co., Ltd. (TCI)
• LGC Science Group Holdings Limited
• AAT Bioquest, Inc.
• Thermo Fisher Scientific, Inc.
• Merck KGaAGet Free Sample Report –https://bisresearch.com/requestsample?id=1375type=download
Key Questions Answered in the Report
- What are the major market drivers, challenges, and opportunities in the global phosphoramidite market?
- What are the key development strategies being implemented by the major players to sustain in the competitive market?
- Which is the dominant phosphoramidite by type developed by the leading and emerging players?
- What is the impact of COVID-19 on the global phosphoramidite market?
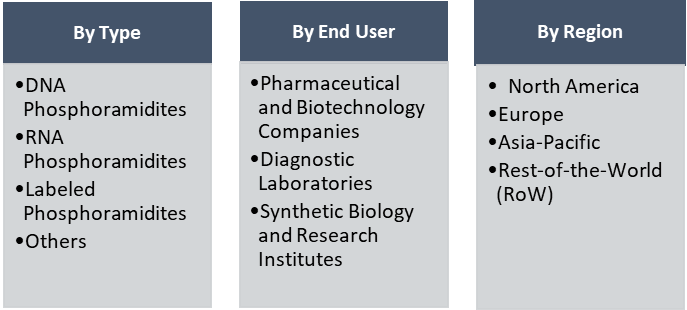
- How is each segment of the market expected to grow during the forecast period from 2022 to 2032? Following are the segments:
- Type (DNA phosphoramidites, RNA phosphoramidites, labeled phosphoramidites, and other phosphoramidites)
- End User (Pharmaceutical and biotechnology companies, diagnostic laboratories, and synthetic biology or research institutes)
- Region (North America, Europe, Asia-Pacific, and Rest-of-the-World)
- Which companies are anticipated to be highly disruptive in the future, and why?
USP of this report
The following can be seen as some of the USPs of this report:
• Extensive competitive benchmarking of key players offering a holistic view of the global phosphoramidite market landscape
• Market ranking analysis based on product portfolio, recent developments, and region-level analysisWho should buy this report?
Established companies involved in the development of phosphoramidite-related products such as oligonucleotides and new entrants who want to focus on the phosphoramidite market would be suitable buyers for this report.
BIS Related Studies
-
Big Data in Healthcare: A Significant Role for Analytics

Big-scale developments in clinical research, the creation of precision medicine, and the creation of clinical decision support tools have all been made possible by the availability of such a large volume of data.
The global big data in healthcare market stands to benefit from growing acceptance for connected devices and digital, data-driven healthcare across the globe. There are efforts being made by stakeholders in the industry to increase the volume of structured data in the healthcare space, which currently is only about 20% of the total data. Additionally, companies are focusing on increasing data interoperability. Data vendors and analytics service providers must focus on developing data cleaning and curating techniques which can aid in the extraction of powerful insights without compromising on data privacy.
According to the latest market intelligence report by BIS Healthcare The global big data in healthcare market report highlights that the market was valued at $32,925.1 million in 2021 and is expected to reach $130,132.1 million by the end of 2031. The market is expected to grow at a CAGR of 13.96% during the forecast period 2022-2031. The growth of the market is expected to be significantly boosted by the growing demand for evidence-based clinical care in developing countries. Technological advancements anticipated from 2022 to 2031 are expected to play a crucial role in deciding the future of the big data in healthcare market and the level of adoption.

Big Data in Healthcare Emerging countries have recently begun to tap into the significant potential of the big data in healthcare market. While the potential is strong, it may take some time to achieve a sizeable adoption rate as the entire data capture process may need to be overhauled in case some countries where basic patient data collection is still done on paper. The COVID-19 pandemic has played a key role in increasing the adoption rate of connected devices and also brought a of urgency towards achieving higher adoption rates. The next decade can be significantly transformative for the healthcare industry across the globe due to the increased focus on data-driven healthcare alone.
The prominent players in this market are Active Health Management, Allscripts Healthcare Solutions, Inc., Cerner Corporation, Epic Systems Corporation, eClinical Works, General Electric Company, Siemens Healthineers AG, JLL
Partners, Verana Health, ConcertAI, LynxCare, Abacus Insights, Milagro AI, Datavant, and Clarify Health Solutions.Among many other things, the projected period is expected to see analytics services grow at the fastest rate. Financial and clinical analytics are further divided into categories in analytics. A few of the causes that contributed to financial insurance holding such a sizable market share include early applications of analytics for enhancing financial outcomes by monitoring performances across revenue cycle management, insurance claim handling, and data detection. Furthermore, significant organizations are becoming interested in clinical analytics.
Globally, North America is dominating the big data in healthcare market because the continent was one of the first to adopt the technology. APAC is also predicted to increase at the greatest rate over the projection period due to the region’s development of big data infrastructure. Big data usage in healthcare has increased globally due to a number of factors, including the promotion of interoperable shared electronic health records (EHRS), proper security measures to safeguard sensitive data, and the development of a more cooperative research environment. To enable big data to reach a company in the healthcare sector, it is necessary to strengthen the communication between the existing databases. In order to centralize patient data over time and across numerous clinics, national data and international platforms must be built.
BIS Healthcare has conducted deep research on the global big data in the healthcare market and compiled the observation and insight in this report. The market intelligence report aims at providing an in-depth analysis of the key development strategies, marketing strategies and market trend dynamics which include drivers, restraints, and opportunities prevailing in the industry.
-
3D Protein Structure Analysis Market is Estimated Drive the Industry Growth Across World in Coming Year

One of the primary objectives of molecular biology research is to identify the structure of proteins. Protein complex analysis requires a thorough research of proteins’ structure and function because they are found in complicated biological samples. The structure of protein complexes can be ascertained using contemporary protein complex analysis methods. Many studies are currently being conducted employing cryo-electron microscopy (EM), small angle X-ray scattering (SAXS), nuclear magnetic resonance (NMR) spectroscopy, and X-ray crystallography to describe and understand molecular structures and molecular recognition mechanisms. Equipment for 3D protein structure analysis is available from reputable manufacturers including Bruker Corporation, JEOL Ltd., and Spectris plc depending on what is required. These tools can meet the laboratory’s throughput requirements.
In 2021, the global 3D protein structure analysis market was valued at $1,017.2 million, and it is expected to reach $2,676.6 million by 2032, growing at a CAGR of 9.32% during the forecast period 2022-2032. The growth in the global 3D protein structure analysis market is expected to be driven by technological advancements in equipment for protein structure analysis and by an increase in RD expenditure to accelerate protein structure analysis.

Impact
• The presence of major equipment providers of 3D protein structure analysis has a major impact on the market. For instance, in April 2022, NJ Biopharmaceuticals LLC and JEOL Ltd. announced their collaboration to bring innovative drug discovery platform solutions using JEOL’s 800 MHz NMR. With the help of this partnership, JEOL Ltd. aspires to grow its 3D protein structure analysis base and increase its existing product line.
• In August 2020, Thermo Fisher Scientific Inc., a global leader in providing scientific services, announced that it would facilitate access to cryo-electron microscopy (cryo-EM) by connecting pharmaceutical and biotechnology companies with contract research organizations (CROs) that provide start-up packages for this game-changing technology as a service.Recent Developments in Global 3D Protein Structure Analysis Market
• In February 2022, JEOL Ltd. announced the development of a new cold field emission cryo-electron microscope (cryo-EM), the CRYO ARM 200 II (JEM-Z200CA), dedicated to single particle analysis of proteins.
• In November 2021, JEOL Ltd. announced the availability of “ECZ Luminous” nuclear magnetic resonance console (JNM-ECZL series). This product is the next step in spectrometer miniaturization and extended performance through state-of-the-art digital and high-frequency technologies.
• In October 2020, Thermo Fisher Scientific Inc. introduced two ground-breaking imaging filters, i.e., the Thermo Scientific Selectris Imaging Filter and the Thermo Scientific Selectris X Imaging Filter, which elevated cryo-electron microscopy (cryo-EM) to a new level by allowing users to view proteins at true atomic resolution.
• In April 2021, Thermo Fisher Scientific Inc. unveiled the Thermo Scientific E-CFEG, a significant accessory for the Thermo Scientific Krios Cryo-TEM and the company’s new cold field emission gun. In comparison to other commercially available technologies, the E-four CFEG’s key components work together to achieve single particle analysis (SPA) resolution levels that are unprecedented.Global 3D Protein Structure Analysis Market Segments
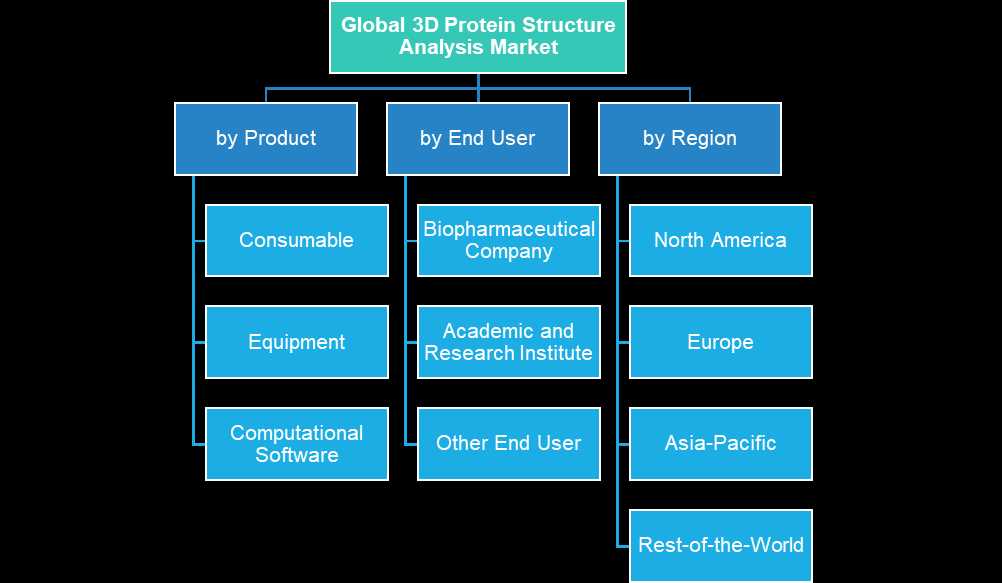
Demand – Drivers and Limitations
Following are the demand drivers for the global 3D protein structure analysis market:
• Technological Advancements in Equipment for Protein Structure Analysis
• Increase in RD Expenditure in Drug Discovery and Development
• Rising Focus on Automation and Miniaturization in X-Ray Crystallography WorkflowThe market is expected to face some limitations too due to the following challenges:
• Certain Instrument Limitations Pertaining to 3D Protein Structure Analysis
• Lack of Qualified Personnel
• High Cost and Time-Consuming MethodKey Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts, analyzing company coverage, product portfolio, and market penetration.
Some of the prominent names established in this market are:
• Bruker Corporation
• JEOL Ltd.
• Spectris plc
• Thermo Fisher Scientific Inc.
• Merck KGaA
• Schrodinger, Inc.
• Molecular Dimensions
• Arinax Scientific Instrumentation.
• Cambridge Isotope Laboratories, Inc.
• HAMPTON RESEARCH CORP.
• DNASTAR
• RosettaCommons.org
• Rigaku Corporation
• Dassault Systemes
• Jena Bioscience GmbHGet Free Sample Report – https://bisresearch.com/requestsample?id=1364type=download
Key Questions Answered in the Report
- What are the major market drivers, challenges, and opportunities and their respective impacts in the global 3D protein structure analysis market?
- What is the potential impact of technological advancements among the key end users, such as biopharmaceutical companies and academic and research institutes?
- What is the current market demand along with future expected demand for the global 3D protein structure analysis market?
- What are the key development strategies that have been implemented by the major players in order to sustain the competitive market?
- Which potential entry barriers are expected to be faced by the companies willing to enter the global 3D protein structure analysis market?
- How is each segment of the market expected to grow during the forecast period from 2022 to 2032 based on each segment?
- Following are the segment types:
- Product (Consumable, Equipment, and Software)
- End User (Biopharmaceutical Company, Academic and Research Institute, Other End User)
- Region (North America, Europe, Asia-Pacific, and Rest-of-the-World (RoW))
- Who are the leading players with significant offerings to the global 3D protein structure analysis market, and what is the expected market dominance for each of these leading players?
- Which emerging companies are anticipated to be highly disruptive in the future, and what are their key strategies for sustainable growth in the global 3D protein structure analysis market?
- Which companies are anticipated to be highly disruptive in the future, and why?
- Who are the leading computational software providers with significant offerings in the global 3D protein structure analysis market?
- Who are the major equipment manufacturers for different technologies in the global 3D protein structure analysis market?
- What are the growth opportunities for the companies in the region of their operation?
BIS Related Studies
-
Subscribe
Subscribed
Already have a WordPress.com account? Log in now.